Diindeno-fused bischrysene, a new diindeno-based polycyclic hydrocarbon (PH), was synthesized and characterized. It was elucidated in detailed experimental and theoretical studies that this cyclopenta-fused PH possesses an open-shell singlet biradical structure in the ground state and exhibits high stability under ambient conditions (t(1/2) = 39 days). The crystal structure unambiguously shows a novel saddle-shaped pi-conjugated carbon skeleton due to the steric hindrance of the central cove-edged bischrysene unit. UV/Vis spectral measurements revealed that the title molecule has a very narrow optical energy gap of 0.92 eV, which is consistent with the electrochemical analysis and further supported by density functional theory (DFT) calculations.
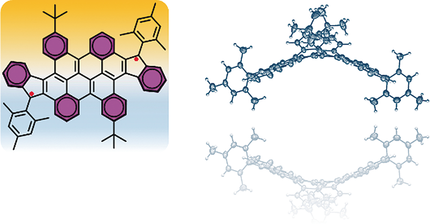
Diindeno-fused bischrysene, a new diindeno-based polycyclic hydrocarbon (PH), was synthesized and characterized. It was elucidated in detailed experimental and theoretical studies that this cyclopenta-fused PH possesses an open-shell singlet biradical structure in the ground state and exhibits high stability under ambient conditions (t(1/2) = 39 days). The crystal structure unambiguously shows a novel saddle-shaped pi-conjugated carbon skeleton due to the steric hindrance of the central cove-edged bischrysene unit. UV/Vis spectral measurements revealed that the title molecule has a very narrow optical energy gap of 0.92 eV, which is consistent with the electrochemical analysis and further supported by density functional theory (DFT) calculations.
