Photocatalytic degradation of organic components in water by means of TiO2 nanosuspensions under ultraviolet (UV) irradiation represents an efficient method for water purification. In the present paper, a modeling approach is proposed to simulate the involved kinetic processes based on the Langmuir-Hinshelwood mechanism. The extended model also includes the formation of intermediate organic components either by an incremental degradation mechanism or by a fragmentation mechanism. Model parameters were estimated from comparison with experimental findings. To demonstrate these models, adsorption and degradation experiments were performed using the antibiotic ciprofloxacin and the dye methylene blue as organic compounds and TiO2 and ZnO as photocatalytic materials. By comparing our simulations with concentration measurements, we found that the adsorption of organic molecules on the surface of the photocatalyst was rate determining at an irradiation intensity of about 20 W m(-2). The derived adsorption rates for ZnO were considerably higher than those for TiO2. The calculated concentration evolution of intermediates as well as the TOC evolution are discussed for different model assumptions with respect to their desorption rates from the photocatalyst surface. (C) 2014 Elsevier B.V. All rights reserved.
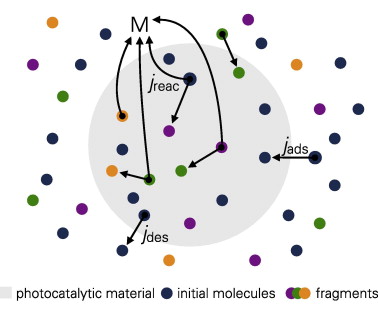
Photocatalytic degradation of organic components in water by means of TiO2 nanosuspensions under ultraviolet (UV) irradiation represents an efficient method for water purification. In the present paper, a modeling approach is proposed to simulate the involved kinetic processes based on the Langmuir-Hinshelwood mechanism. The extended model also includes the formation of intermediate organic components either by an incremental degradation mechanism or by a fragmentation mechanism. Model parameters were estimated from comparison with experimental findings. To demonstrate these models, adsorption and degradation experiments were performed using the antibiotic ciprofloxacin and the dye methylene blue as organic compounds and TiO2 and ZnO as photocatalytic materials. By comparing our simulations with concentration measurements, we found that the adsorption of organic molecules on the surface of the photocatalyst was rate determining at an irradiation intensity of about 20 W m(-2). The derived adsorption rates for ZnO were considerably higher than those for TiO2. The calculated concentration evolution of intermediates as well as the TOC evolution are discussed for different model assumptions with respect to their desorption rates from the photocatalyst surface. (C) 2014 Elsevier B.V. All rights reserved.
