Pharmaceuticals have become an important public health issue as environmental pollutants over the last years. After ingestion, pharmaceuticals are partly excreted unchanged. They can reach the wastewater treatment plant (WWTP) via the sewer network. Because the conventional treatments are ineffective in their removal, new methods should be approached, for example semiconductor photocatalysis. Several of the hitherto published studies analyzed the degradation of model pollutants but for the degradation of pharmaceuticals in unspiked real wastewater further investigations are required. Therefore, we want to focus on the removal of pharmaceuticals in an actual effluent from a WWTP and investigate the effluent background effect. This study shows the heterogeneous photocatalytic degradation of 14 pharmaceuticals with initial concentrations C-i > 0.3 mu g L (1) present in a WWTP effluent. We found that UVA (1.5 mW cm (2), intensity peak at 365 nm) irradiation of TiO2 P25 (A(s) = 56 m(2) g (1)) or ZnO (A(s) = 5.23 m(2) g (1)) nanoparticles leads to considerable degradation of the analyzed pharmaceuticals. With ZnO nanoparticles, 40 min UVA irradiation was sufficient to degrade over 95% of these pharmaceuticals (k(app) = 8.6 x 10 (2) s (1)). Using TiO2 P25 on the other hand, it would take more than six times longer to reach the same level (k(app) = 1.4 x 10 (2) s (1)). Carbamazepine dissolved in millipore water served as a comparison model. Also in this system ZnO presents faster degradation. (C) 2015 Elsevier Ltd. All rights reserved.
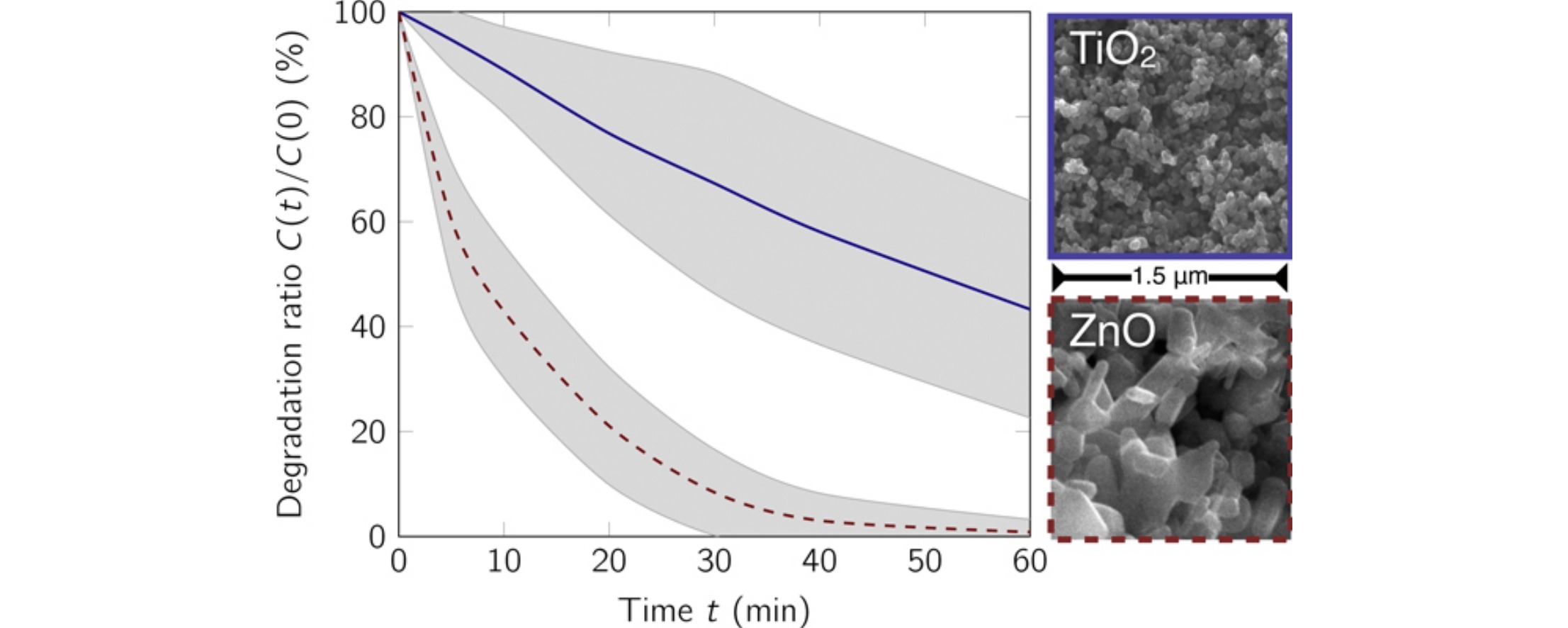
Pharmaceuticals have become an important public health issue as environmental pollutants over the last years. After ingestion, pharmaceuticals are partly excreted unchanged. They can reach the wastewater treatment plant (WWTP) via the sewer network. Because the conventional treatments are ineffective in their removal, new methods should be approached, for example semiconductor photocatalysis. Several of the hitherto published studies analyzed the degradation of model pollutants but for the degradation of pharmaceuticals in unspiked real wastewater further investigations are required. Therefore, we want to focus on the removal of pharmaceuticals in an actual effluent from a WWTP and investigate the effluent background effect. This study shows the heterogeneous photocatalytic degradation of 14 pharmaceuticals with initial concentrations C-i > 0.3 mu g L (1) present in a WWTP effluent. We found that UVA (1.5 mW cm (2), intensity peak at 365 nm) irradiation of TiO2 P25 (A(s) = 56 m(2) g (1)) or ZnO (A(s) = 5.23 m(2) g (1)) nanoparticles leads to considerable degradation of the analyzed pharmaceuticals. With ZnO nanoparticles, 40 min UVA irradiation was sufficient to degrade over 95% of these pharmaceuticals (k(app) = 8.6 x 10 (2) s (1)). Using TiO2 P25 on the other hand, it would take more than six times longer to reach the same level (k(app) = 1.4 x 10 (2) s (1)). Carbamazepine dissolved in millipore water served as a comparison model. Also in this system ZnO presents faster degradation. (C) 2015 Elsevier Ltd. All rights reserved.
