Chirality-induced spin selectivity (CISS) is a recently discovered effect, whose precise microscopic origin has not yet been fully elucidated; it seems, however, clear that spin-orbit interaction plays a pivotal role. Various model Hamiltonian approaches have been proposed, suggesting a close connection between spin selectivity and filtering and helical symmetry. However, first-principles studies revealing the influence of chirality on the spin polarization are missing. To clearly demonstrate the influence of the helical conformation on the spin polarization properties, we have carried out spin-dependent Density-Functional Theory (DFT) system based transport calculations for a model molecular system. It consists of alpha-helix and beta-strand conformations of an oligo-glycine peptide, which is bonded to a nickel electrode and to a gold electrode in a two-terminal setup, similar to a molecular junction or a local probe, for example, in STM or AFM configurations. We have found that the alpha-helix conformation displays a spin polarization, calculated through the intrinsic magneto-resistance of the junction, about 100-1000 times larger than the linear beta-strand, clearly demonstrating the crucial role played by the molecular helical geometry on the enhancement of spin polarization associated with the CISS effect.
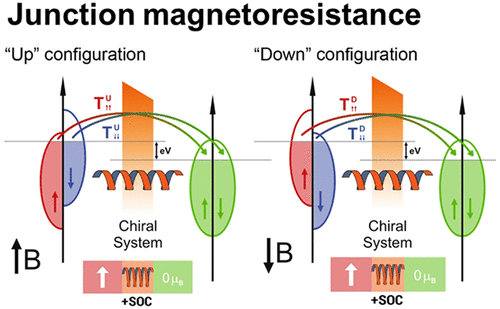
Chirality-induced spin selectivity (CISS) is a recently discovered effect, whose precise microscopic origin has not yet been fully elucidated; it seems, however, clear that spin-orbit interaction plays a pivotal role. Various model Hamiltonian approaches have been proposed, suggesting a close connection between spin selectivity and filtering and helical symmetry. However, first-principles studies revealing the influence of chirality on the spin polarization are missing. To clearly demonstrate the influence of the helical conformation on the spin polarization properties, we have carried out spin-dependent Density-Functional Theory (DFT) system based transport calculations for a model molecular system. It consists of alpha-helix and beta-strand conformations of an oligo-glycine peptide, which is bonded to a nickel electrode and to a gold electrode in a two-terminal setup, similar to a molecular junction or a local probe, for example, in STM or AFM configurations. We have found that the alpha-helix conformation displays a spin polarization, calculated through the intrinsic magneto-resistance of the junction, about 100-1000 times larger than the linear beta-strand, clearly demonstrating the crucial role played by the molecular helical geometry on the enhancement of spin polarization associated with the CISS effect.
