Here we propose a platform for the detection of unlabeled human ?-thrombin down to the picomolar range in a fluorescence-based aptamer assay. In this concept, thrombin is captured between two different thrombin binding aptamers, TBA1 (15mer) and TBA2 (29mer), each labeled with a specific fluorescent dye. One aptamer is attached to the surface, the second one is in solution and recognizes surface-captured thrombin. To improve the limit of detection and the comparability of measurements, we employed and compared two approaches to pattern the chip substrate: microcontact printing of organosilanes onto bare glass slides, and controlled printing of the capture aptamer TBA1 in arrays onto functionalized glass substrates using a nanoplotter device. The parallel presence of functionalized and control areas acts as an internal reference. We demonstrate that both techniques enable the detection of thrombin concentrations in a wide range from 0.02 to 200 nM with a detection limit at 20 pM. Finally, the developed method could be transferred to any substrate to probe different targets that have two distinct possible receptors without the need for direct target labeling.
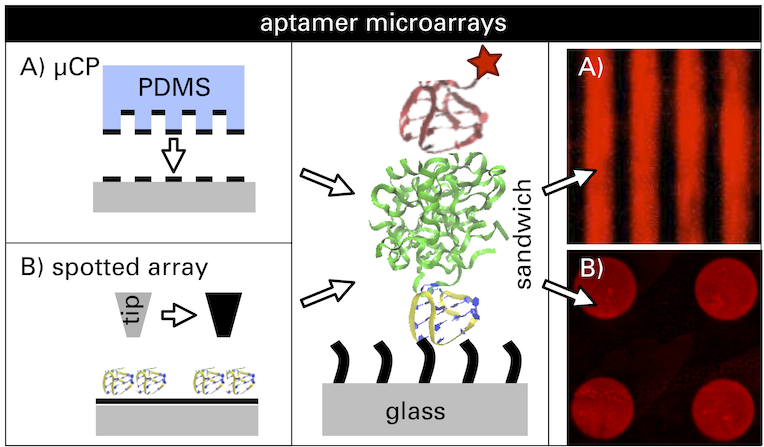
Here we propose a platform for the detection of unlabeled human ?-thrombin down to the picomolar range in a fluorescence-based aptamer assay. In this concept, thrombin is captured between two different thrombin binding aptamers, TBA1 (15mer) and TBA2 (29mer), each labeled with a specific fluorescent dye. One aptamer is attached to the surface, the second one is in solution and recognizes surface-captured thrombin. To improve the limit of detection and the comparability of measurements, we employed and compared two approaches to pattern the chip substrate: microcontact printing of organosilanes onto bare glass slides, and controlled printing of the capture aptamer TBA1 in arrays onto functionalized glass substrates using a nanoplotter device. The parallel presence of functionalized and control areas acts as an internal reference. We demonstrate that both techniques enable the detection of thrombin concentrations in a wide range from 0.02 to 200 nM with a detection limit at 20 pM. Finally, the developed method could be transferred to any substrate to probe different targets that have two distinct possible receptors without the need for direct target labeling.
