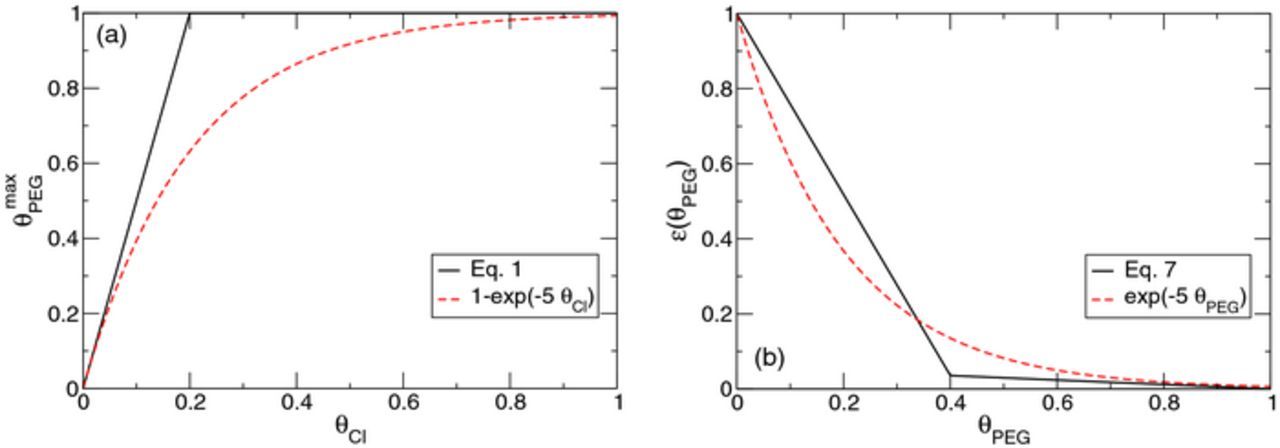
Additives play an important role in electrochemical deposition and understanding their working mechanism is a great challenge. In cyclic voltammetry measurements of copper deposition, hysteresis is ubiquitously observed. Correct prediction of hysteresis behavior is an important test for deposition models. In previous models, including poly(ethylene glycol) (PEG) and chloride ions as additives, a common assumption to explain the hysteresis is the consumption of additives during copper deposition. However, second-ion mass spectrometry measurements often detected comparatively low levels of impurities in deposits. Therefore, we propose here an alternative mechanism for explaining hysteresis curves without invoking additive consumption. Essential ingredients of our models are: (i) a strongly nonlinear dependence of the maximal possible PEG coverage on the chloride coverage on the copper surface, (ii) a nonlinear dependence of the deposition current on the PEG surface coverage, and (iii) an additional activation of the desorption of additives with increasing copper deposition current. We demonstrate that our model reproduces characteristic features of cyclic voltammograms measured under vastly different conditions and exhibiting pronounced hysteresis. Furthermore, simulations are compared well to PEG adsorption/desorption experiments with varying additive concentrations. The proposed model may serve to describe deposition situations with negligible additive consumption. (C) 2017 The Electrochemical Society. All rights reserved.
Additives play an important role in electrochemical deposition and understanding their working mechanism is a great challenge. In cyclic voltammetry measurements of copper deposition, hysteresis is ubiquitously observed. Correct prediction of hysteresis behavior is an important test for deposition models. In previous models, including poly(ethylene glycol) (PEG) and chloride ions as additives, a common assumption to explain the hysteresis is the consumption of additives during copper deposition. However, second-ion mass spectrometry measurements often detected comparatively low levels of impurities in deposits. Therefore, we propose here an alternative mechanism for explaining hysteresis curves without invoking additive consumption. Essential ingredients of our models are: (i) a strongly nonlinear dependence of the maximal possible PEG coverage on the chloride coverage on the copper surface, (ii) a nonlinear dependence of the deposition current on the PEG surface coverage, and (iii) an additional activation of the desorption of additives with increasing copper deposition current. We demonstrate that our model reproduces characteristic features of cyclic voltammograms measured under vastly different conditions and exhibiting pronounced hysteresis. Furthermore, simulations are compared well to PEG adsorption/desorption experiments with varying additive concentrations. The proposed model may serve to describe deposition situations with negligible additive consumption. (C) 2017 The Electrochemical Society. All rights reserved.
