Graphene is extremely sensitive to its surrounding environment. In fact, any modification on its surface, such as an adsorption of a molecule, can change its properties. The conductivity of graphene is a crucial parameter to be examined for potential graphene-based applications, especially biosensors. In this paper, we have investigated the effects of non-covalent functionalization based on π–π interaction, using 1-pyrenebutyric acid N-hydroxysuccinimide ester (PBASE), on the conductivity of graphene-based electrodes by electrochemical techniques. Graphene layers were obtained by chemical vapor deposition (CVD) and characterized by Raman spectroscopy, Scanning Electron Microscopy (SEM), and X-Ray Diffraction (XRD). The results demonstrate the synthesis of a high quality and continuous monolayer graphene with an I2D/IG ratio = 2.71 and ID = 0. After the functionalization of graphene-based electrode with PBASE, the electrochemical analyses confirmed a p-doping effect existence influencing the conductivity by increasing the charge transfer resistance Rct from 816.5 to 2213 Ω. Furthermore, increasing the concentration of PBASE from 5 mM to 10 mM did not affect the conductivity as the Rct did not change. Solvent adsorption was found to be the main cause of the decrease in conductivity during the functionalization process. This study highlights the effect of non-covalent π-π stacking of PBASE on graphene to tune the electrical properties of graphene through functionalization processes for a better performance for their use as biosensors.
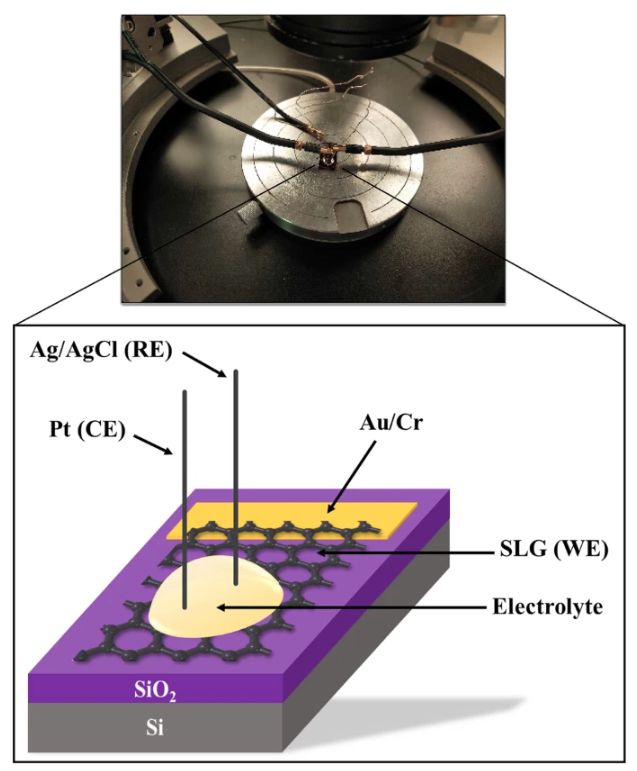
Graphene is extremely sensitive to its surrounding environment. In fact, any modification on its surface, such as an adsorption of a molecule, can change its properties. The conductivity of graphene is a crucial parameter to be examined for potential graphene-based applications, especially biosensors. In this paper, we have investigated the effects of non-covalent functionalization based on π–π interaction, using 1-pyrenebutyric acid N-hydroxysuccinimide ester (PBASE), on the conductivity of graphene-based electrodes by electrochemical techniques. Graphene layers were obtained by chemical vapor deposition (CVD) and characterized by Raman spectroscopy, Scanning Electron Microscopy (SEM), and X-Ray Diffraction (XRD). The results demonstrate the synthesis of a high quality and continuous monolayer graphene with an I2D/IG ratio = 2.71 and ID = 0. After the functionalization of graphene-based electrode with PBASE, the electrochemical analyses confirmed a p-doping effect existence influencing the conductivity by increasing the charge transfer resistance Rct from 816.5 to 2213 Ω. Furthermore, increasing the concentration of PBASE from 5 mM to 10 mM did not affect the conductivity as the Rct did not change. Solvent adsorption was found to be the main cause of the decrease in conductivity during the functionalization process. This study highlights the effect of non-covalent π-π stacking of PBASE on graphene to tune the electrical properties of graphene through functionalization processes for a better performance for their use as biosensors.
