High sensitivity and selectivity are never-ending points of interest in the gas sensing field. Herein, the novel functionalized N-doped graphitic carbon is derived from Zn-MOF by modulating the pyrolysis temperature toward H2S sensing application. The results demonstrate excellent sensing performance toward H2S gas with a limit of detection (LOD) of 56.9 ppb, faster response and recovery time (18 and 29 s), and high selectivity with a 20-fold response difference than other interfering gases. The expected stability with stable multiple consecutive responses and a strong response toward 1 ppm of H2S after 4 months were reached. Functionalized groups pyridinic nitrogen (PD-N) and pyrrolic nitrogen (PR-N) that make MOF-derived carbon stand out in H2S gas sensing are mainly attributed to dual active sites: (i) N–C bonds on graphitic carbon undergo surface redox reactions, forming oxidized carbon species (C═O or C═S), and (ii) PD/PR-N-Zn coordination centers facilitate the formation of SO42–-based surface complexes through reaction with H2S and adsorbed oxygen. Notably, DFT calculation was employed to confirm both PR-N and PD-N bonding with zinc, yielding the largest charge transfer and binding energy among simulated factors, which attributes to the generation of significant sensing performance for H2S. Consequently, this work will provide a novel strategy for the advancement of gas sensing applications.
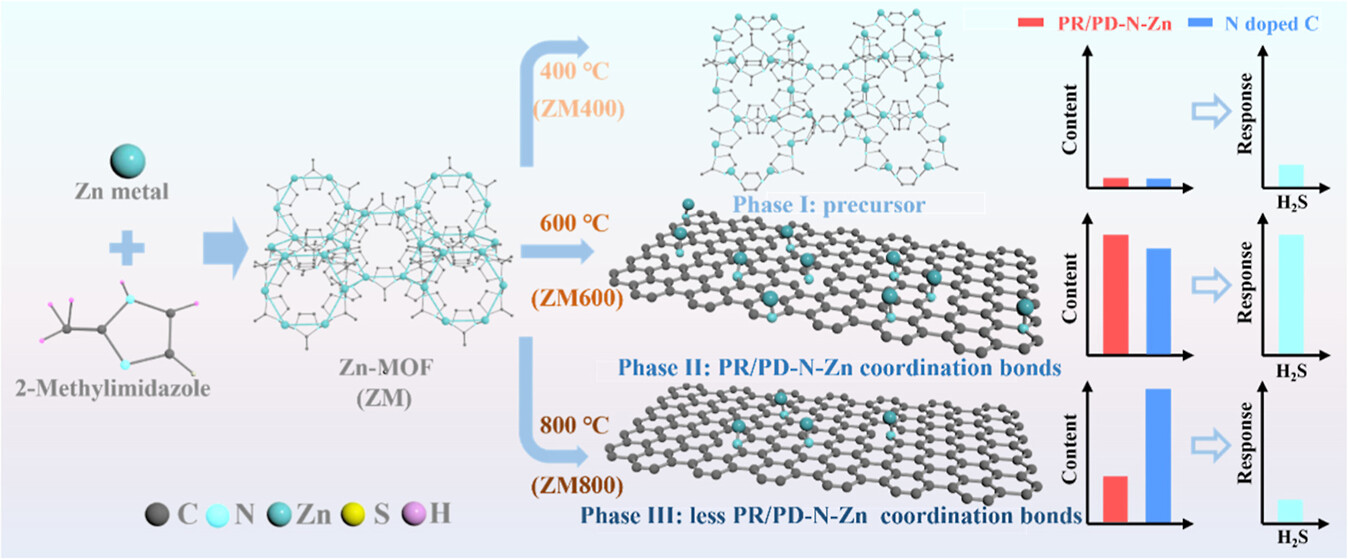
High sensitivity and selectivity are never-ending points of interest in the gas sensing field. Herein, the novel functionalized N-doped graphitic carbon is derived from Zn-MOF by modulating the pyrolysis temperature toward H2S sensing application. The results demonstrate excellent sensing performance toward H2S gas with a limit of detection (LOD) of 56.9 ppb, faster response and recovery time (18 and 29 s), and high selectivity with a 20-fold response difference than other interfering gases. The expected stability with stable multiple consecutive responses and a strong response toward 1 ppm of H2S after 4 months were reached. Functionalized groups pyridinic nitrogen (PD-N) and pyrrolic nitrogen (PR-N) that make MOF-derived carbon stand out in H2S gas sensing are mainly attributed to dual active sites: (i) N–C bonds on graphitic carbon undergo surface redox reactions, forming oxidized carbon species (C═O or C═S), and (ii) PD/PR-N-Zn coordination centers facilitate the formation of SO42–-based surface complexes through reaction with H2S and adsorbed oxygen. Notably, DFT calculation was employed to confirm both PR-N and PD-N bonding with zinc, yielding the largest charge transfer and binding energy among simulated factors, which attributes to the generation of significant sensing performance for H2S. Consequently, this work will provide a novel strategy for the advancement of gas sensing applications.
